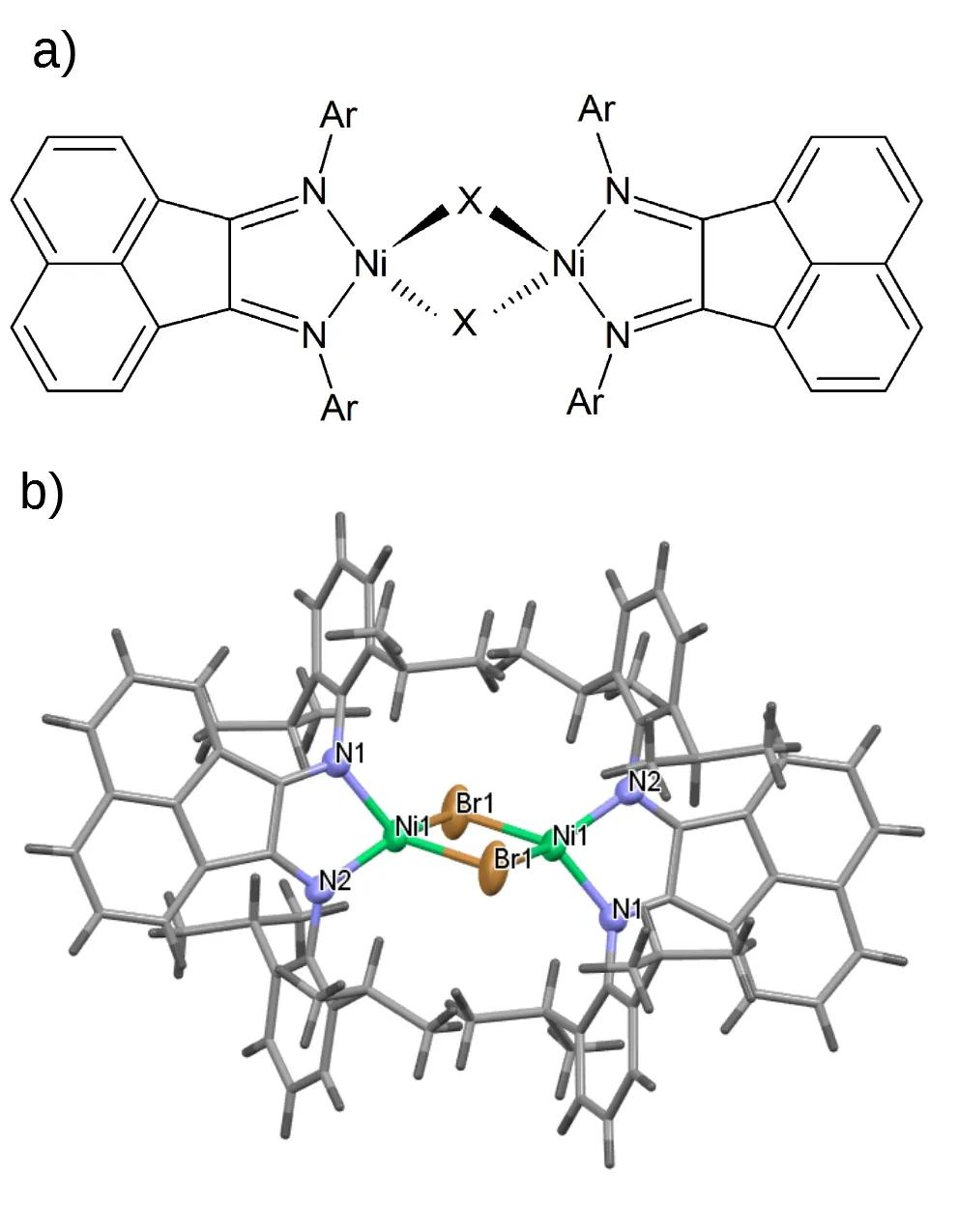
Studies on molecular systems with transition metals and lanthanides have become an important part of our research. This topic has been strongly promoted by our long-term cooperation with Dr. Rustem Zairov and his colleagues based at the A.E. Arbuzov Institute of Organic and Physical Chemistry, KazSC RAS, in Kazan. Within this cooperation, we have been recently dealing with transition metal complexes that comprise 1,2-bis(arylimino)acenaphthenes (bians), see, e.g. the halogen-bridged dinuclear Ni(I) complexes in Fig. 1. The bians and related molecules belong to redox-active or so-called non-innocent ligands, i.e., ligands with an a priori non-obvious and potentially variable oxidation state that can stabilize different oxidation states of metal centres. Specifically, the 14-electron π system of a neutral bian molecule can reversibly accept up to four additional electrons. Other molecular systems studied recently in our group involve for example a dinuclear complex of Cr(II) stabilized by bulky alkylcyclopentadienyl.
As regards magnetic studies on molecular compounds, we combine both experimental approach and calculations from first principles, above all SQUID magnetometry, PPMS measurements, and DFT calculations. We employ also 57Fe Mössbauer spectroscopy, which is available to us in cooperation with the Laboratory of Mössbauer spectroscopy at the MFF UK. Importantly, we are capable to handle and carry out magnetic measurements on highly sensitive samples that have to be mounted in a glovebox under strictly inert atmosphere (< 1ppm O2, <1 ppm H2O).
Recently, we have established within our department a fully equipped chemical laboratory with a glovebox, a Schlenk line and special equipment for the synthesis of highly sensitive coordination/organometallic compounds. Our synthetic efforts are now directed to novel transition metal complexes, including redox-active ligands, and single-molecule magnets.
References: [1] V.V. Khrizanforova, R.R. Fayzullin, V.I. Morozov, I.F. Gilmutdinov, A.N. Lukoyanov, O.N. Kataeva, T.P. Gerasimova, S.A. Katsyuba, I.L. Fedushkin, K.A. Lyssenko, Y.H. Budnikova, One-Electron Reduction of Acenaphthene-1,2-Diimine Nickel(II) Complexes, Chem.-Asian J., 14 (2019) 2979-2987. DOI: https://doi.org/10.1002/asia.201900677