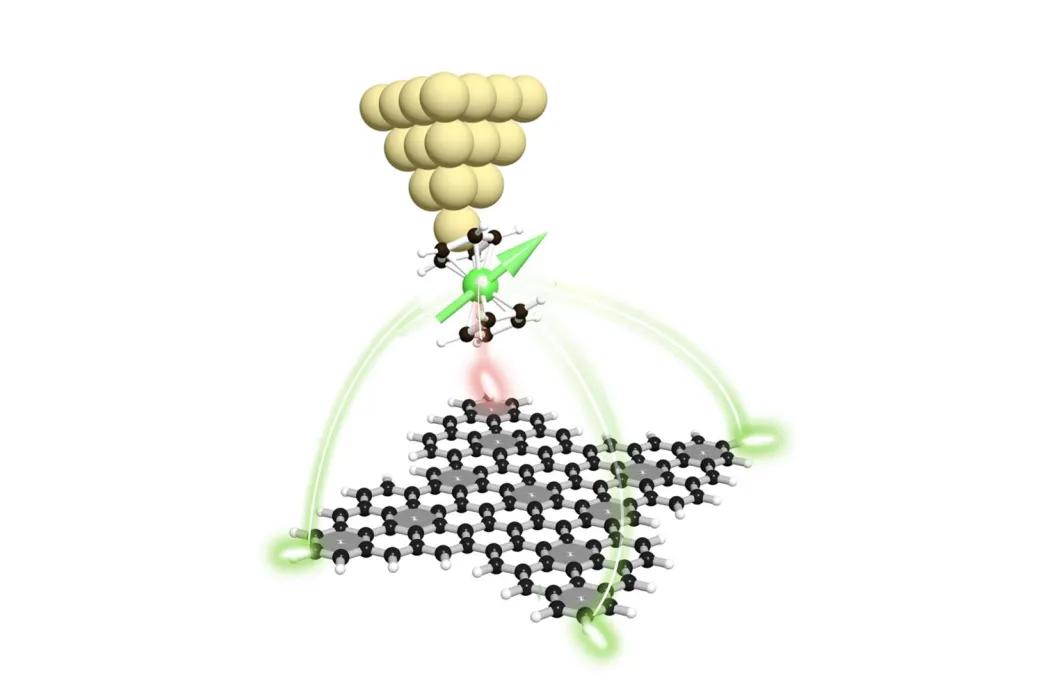
For the first time, an international team of scientists has successfully developed a polyradical nanographene by combining two concepts: pi-magnetism formation in nanographene and strong interaction between electrons and topological frustration and they detected the magnetic signal using advanced scanning microscopy and quantum mechanical calculations. Graphene nanoparticles exhibit magnetic properties in certain shapes, making them suitable for information storage and processing in quantum computing.
The paper, published in Nature Chemistry, describes an innovative method to design, prepare and verify the magnetic properties of graphene in the shape of four rounded triangles resembling "butterfly wings". Each of these triangles contains an unpaired pi electron, which is responsible for the magnetic properties.
"Previous approaches were limited to a single magnetic origin, which limited the number of correlated spins or the type of magnetic ordering in the nanographenes. In this work, we were able to combine two approaches for the first time to create this unique magnetic nanographene with four unpaired electrons. Moreover, by combining experimental and theoretical calculations, we were able to provide irrefutable evidence for its magnetic character," says Adam Matěj from the Institute of Physics of the Czech Academy of Sciences and Palacký University in Olomouc.
Recipe for making nanomaterials from Singapore
Nanographene was synthesised by scientists in Singapore on the surface of gold by heating a pre-prepared organic molecule to 600 Kelvin to dehydrogenate and cycle the bonds in the individual 'butterfly wings'. The entire preparation of nanographene had to be carried out in an ultra-high vacuum because the synthesis of highly reactive compounds in solution is problematic.
The traditional image of magnetism is associated with transition metals such as iron, which contain highly spatially localised unpaired electrons. For a long time it was thought that carbon-based materials with strongly delocalised electrons could not have magnetic properties.
However, research in recent years has shown new ways of making magnetic systems based on nanographene structures. This new concept of magnetism is called pi-magnetism, due to the presence of unpaired pi electrons. One of the unsolved challenges of this new class of magnetism remained not only the preparation of nanographenes with a higher number of unpaired electrons, but also the direct experimental verification of their magnetic character.
The unique magnetic properties of nanographene were verified by Czech scientists from Pavel Jelinek's team using scanning tunneling microscopy, which can measure the local magnetic field in a specific part of the molecule thanks to a probe with a nickelcene molecule.
The experimental results determining the electronic structure were confirmed using state-of-the-art quantum chemical computational methods in collaboration with Libor Veis' team at the J. Heyrovsky Institute of Physical Chemistry. "Calculating the electronic structure of molecules with multiple open shells is generally very challenging. However, we have often seen that the computational tools we are developing can solve this problem with great accuracy. In this case too, we can be confident in the conclusions of our study because of the excellent agreement between experimental and theoretical results. Moreover, theoretical calculations often have the advantage of providing information that is not available experimentally, in this case the way in which individual electron spins are strongly entangled," explains Libor Veis.
Scientists from the National University of Singapore, the CATRIN research institute at Palacký University in Olomouc, Nanjing University in China and two institutes of the Czech Academy of Sciences - the Institute of Physics and the J. Heyrovský Institute of Physical Chemistry - participated in the experimental and theoretical verification of the properties of nanographene.
Shaotang Song, Andrés Pinar Solé, Adam Matěj, Guangwu Li, Oleksandr Stetsovych, Diego Soler, Huimin Yang, Mykola Telychko, Jing Li, Manish Kumar, Qifan Chen, Shayan Edalatmanesh, Jiri Brabec, Libor Veis, Jishan Wu, Pavel Jelinek & Jiong Lu
Nat. Chem. (2024).