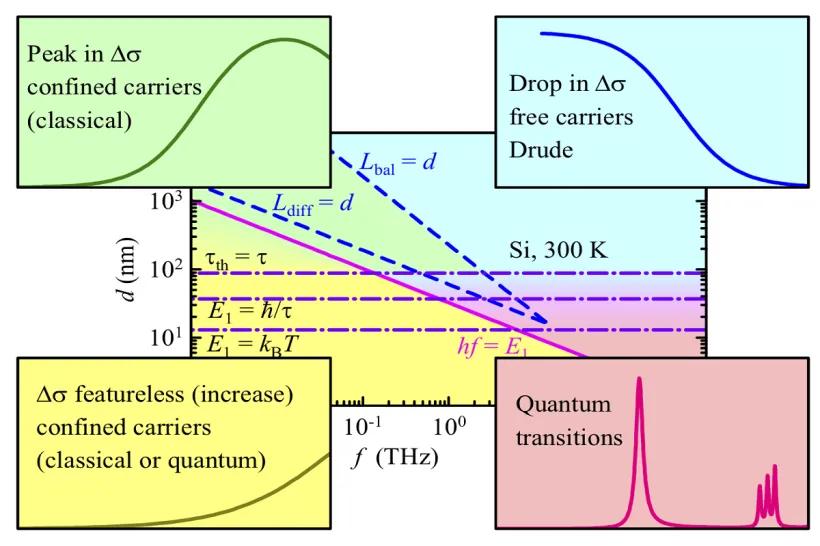
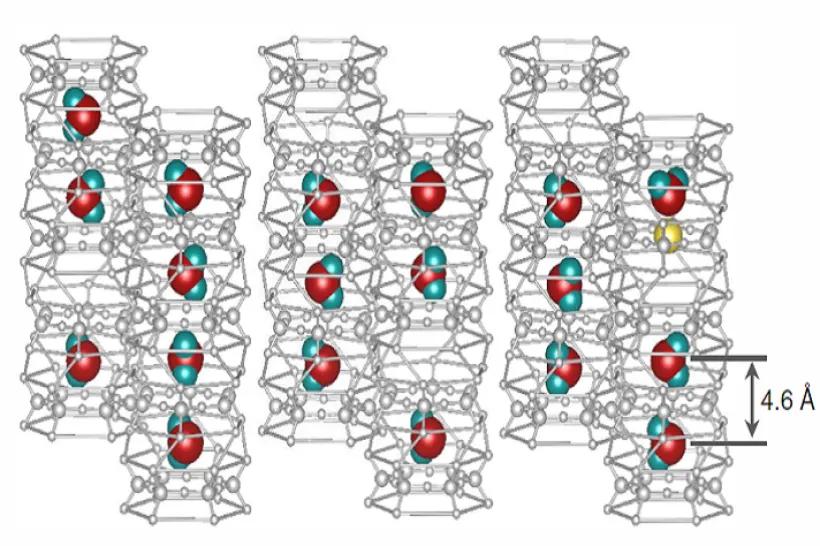
The electronic structure of metals, semiconductors, and complex materials with enhanced electron-electron correlations is studied with quantum-mechanical (ab-initio) methods based on the density functional theory (DFT). A particular attention is given to magnetism, transport properties, and to the influence of lattice imperfections and disorder. Promising materials for applications in spintronics, in information storage, or as strong magnets are investigated in order to analyze the existing experimental data as well as to guide new experiments.
Motivated by technological advances achieved in preparation of nanoscale devices with properties well controlled down to single atoms, we study magnetic behavior of various nanoscale objects, which is often substantially influenced by strong electron correlations. The rich and often intriguing behavior of compounds containing rare-earth metals or actinides (like uranium) represents another research line.
Detailed microscopic understanding of all these materials calls for modern electronic-structure methods and computer codes, as well as for their continued in-house development. For the materials with correlated electrons, we work on the DFT+DMFT framework that builds on material-specific Hubbard models, which are solved by the dynamical mean-field theory (DMFT). For the description of non-periodic structures we develop a real-space computer code that solves the equations of the density functional theory using the finite-elements method.