A new way to control the electronic and magnetic properties of molecules has been discovered by scientists from the Regional Centre of Advanced Technologies and Materials (RCPTM) at Palacký University Olomouc, together with colleagues from the Institutes of Physics (FZU) and Organic Chemistry and Biochemistry (IOCB) of the Czech Academy of Science. Commonly, a change in the electronic configuration of molecules can be induced by the application of external stimuli, such as light, temperature, pressure, and magnetic field. Czech scientists have instead developed a revolutionary way to use weak non-covalent interactions of molecules with the surface of chemically modified graphene. This achievement has been published in the prestigious Nature Communications journal.
“The ability to repeatedly change the electronic structure of molecules and their magnetic properties has been of interest to scientists over the last few decades due to the great potential for application. Switching from one magnetic state to another is, with respect to the small size of molecules, an important step towards developing molecular computers," said Mr Pavel Jelínek from the FZU and RCPTM. Molecular switches are to find the greatest use in nanoelectronics, biology or medicine.
Electrical, optical, or magnetic properties of the molecules, as well as their biological activity, are determined by the arrangement of electrons that move within molecules along precisely given tracks, so-called orbitals. Molecules containing orbitals, occupied by only 1 unpaired electron, display magnetic properties. In contrast, molecules that have two paired electrons on all orbitals are non-magnetic.
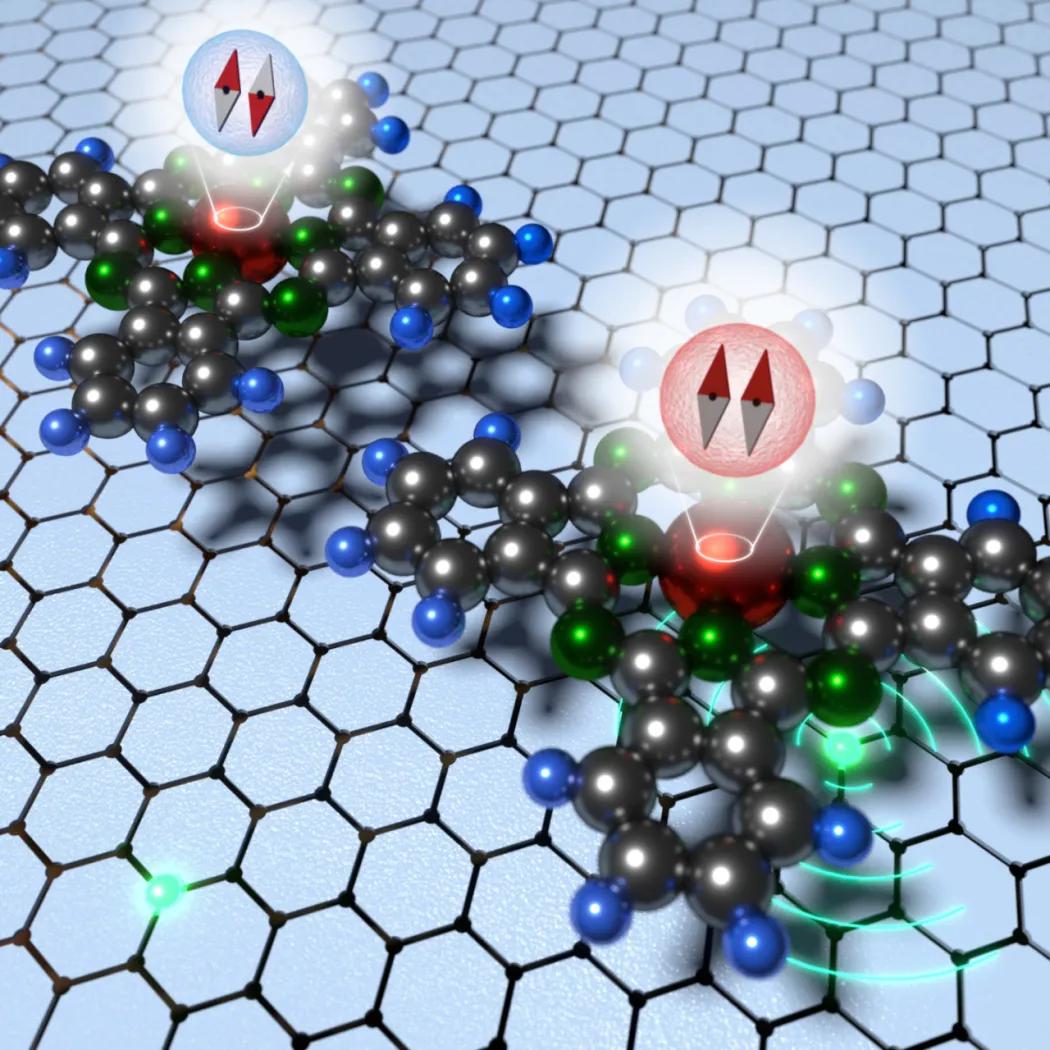
"So far, the switching has been triggered using technology-intensive external sources. A single atomic layer of graphite-graphene was used for the switch, in which we replaced some carbon atoms by nitrogen atoms. By changing the position of the molecule, we are then able to revert from the magnetic state on pure graphene to non-magnetic state in the areas located on nitrogen atoms. Moreover, it was the first time we have managed to observe these changes in the arrangement of electrons in a molecule using atomic forces microscopy. It is undoubtedly great progress in the possibilities of differentiating microscopic scanning techniques," explained Mr Jelínek.
The properties of molecules are typically fine-tuned with covalent chemical modification, leading to a change in the chemical composition of the molecule, i.e. the decomposition of the old molecules and the formation of new chemical bonds in the molecule. In these strong so-called covalent interactions, the electrons which are involved in the coupling are shared. This approach, however, is not applicable to the development of molecular switches, as the chemical modification causes an irreversible change. Czech scientists have therefore decided to use weak non-covalent interactions, although these have not been considered as a possible source for inducing a change in the magnetic state of the molecule.
"It turned out that the use of planar molecules based on porphyrins with a central iron atom leads to rearrangement of electrons when such a molecule is positioned in the vicinity of a nitrogen defect in graphene. By combining both theoretical calculations and experimental measurements, we confirmed that non-covalent interactions between iron and nitrogen atoms are strong enough to disturb the magnetism state and, at the same time, weak enough to allow the transition of the molecule to its magnetic state as soon as the molecule is returned to a pristine graphene surface," says Mr Pavel Hobza, a globally renown expert on non-covalent interaction from the RCPTM and IOCB.
This effortless way of managing the properties of molecules, without their irreversible chemical modification, opens up possibilities in other areas of application. "The electronic structure affects not only the magnetic but also the optical, catalytic, electrical or biological properties of the molecules. Such chemically modified graphene could be used for the development of new optical sensors, photoluminescent materials, catalysts or pharmaceuticals," says Mr Radek Zbořil, Director of RCPTM, listing possible applications. His team has recently authored many other breakthroughs in the field of graphene and magnetism studies. Recently, they reported the first-ever use of graphene to develop non-metallic 2D magnets or the smallest particles of magnetic metals in Nature Communications.
The co-authors of the publication are six other members of Pavel Jelínek’s team from the Thin Layers and Nanostructures Department at the Institute of Physics of the Czech Academy of Sciences. Team members deal with the targeted manipulation of charge and spin in molecular structures on surfaces and their preparation. In doing so, they use imaging techniques such as atomic force microscopy, scanning tunnelling microscopy, electron diffraction, etc.
Doc. Pavel Jelínek graduated from Physics and Materials Engineering at Czech Technical University. He has been with the Institute of Physics since 1999, having worked at other institutions in Spain, the USA and Japan. Mr Jelínek has received recognized awards, including the Academic Premium or the Academy of Sciences Award for outstanding achievements of great scientific significance. His team includes other awardees of significant awards, such as Mr Martin Švec and Mr Prokop Hapal, the winner of the Otto Wichterle Prize.
This report was taken over and extended with the approval of UPOL. Author: Mgr. Martina Šaradínová (UPOL).